
Though they are isomers, maleic acid and fumaric acid have totally different physical properties. These .

Synthesis of fumaric acid. Industrial synthesis of fumaric acid proceeds via
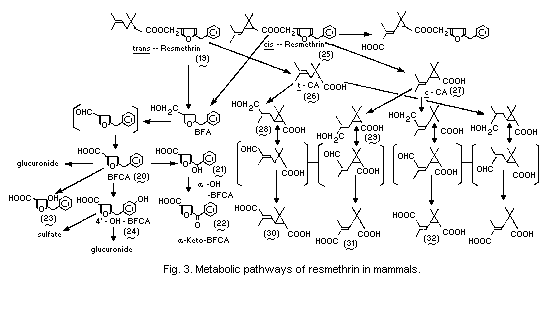
Transformation of a chemicalisomerization, isomerization maleic acid,
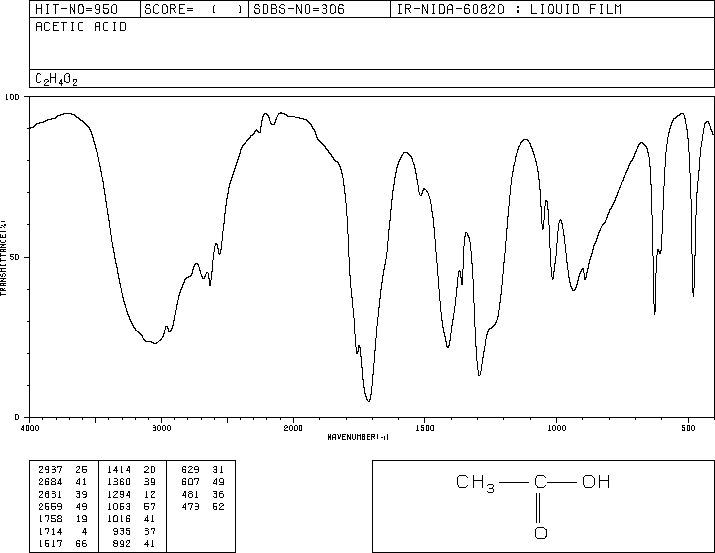
Consider the starting alcohol and glacial acetic acid: are either or both of

Superposition of mgf chemical name, mgf lewis acid readily forming

(4-Oxo-4H-1-benzopyran-2-yl)-oxamic acid, salts and esters anti-allergic

Lead Acid Battery Charger circuit for 6V – 12V. Advertisement

3-aryl-2-hydroxypropionic acid derivatives and analogs as hypoglycemic

Electrons viewed in real time for the first time ever
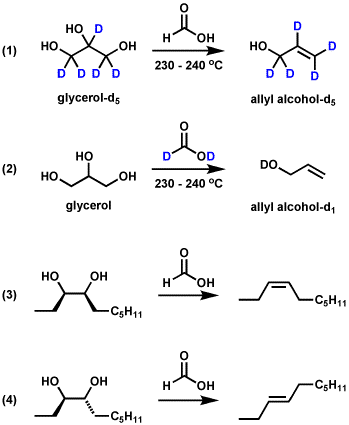
Scheme 3, Mechanism of Formic Acid-Mediated Dideoxygenation

Re: trifluoroacetic acid, I agree that it is a stronger acid.
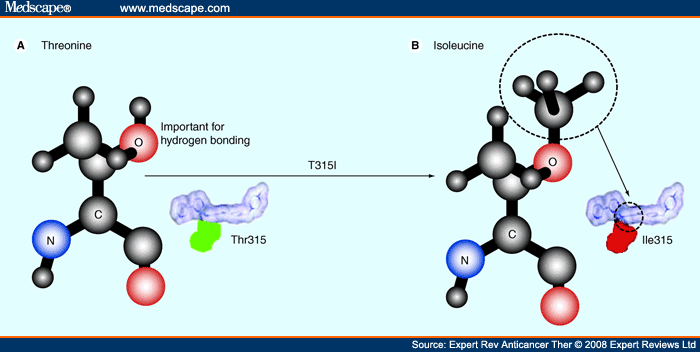
When the amino acid at 315 is substituted to (B) isoleucine from (A)

The pi-bond is presumed to be orthogonal to the benzene electrons.

circuit diagram for lead-acid battery charger.

Solutions of 0.05 M NaOH are added to 10.0 mL of 0.10 M 4-Chlorobenzoic acid
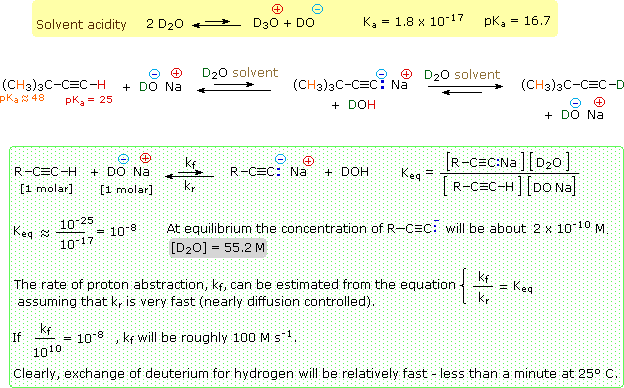
testifying that an acid-base reaction has occurred.

Of bond shift first isomerism due Corn refiners vpr fz smrzshn isomerize

General mechanism of demetalation of 1 by picolinic acid accounting for
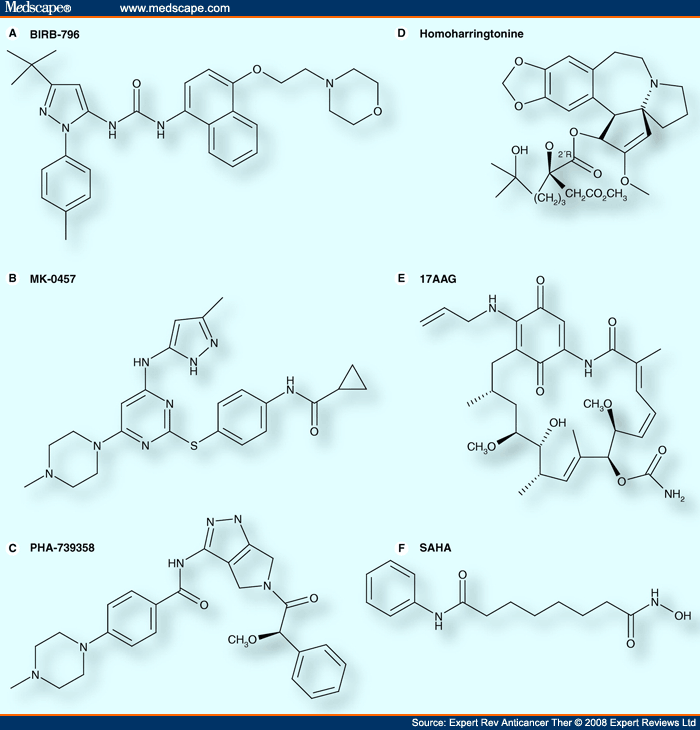
17-AAG: 17-allylaminogeldanamycin; SAHA = Suberoylanilide hydroxamic acid.

assumption--that the effect is due to vinegar or some other acid in the

No comments:
Post a Comment